- Messages
- 2,145
- Reaction score
- 5
- Points
- 28
- Thread Starter
- #101
Re: Gene editing breakthrough could destroy thousands of deadly diseases

In Under the Skin, Scarlett Johanssen plays an alien who seduces men in order to graft their skin to her black mass of a body. In Painted Skin 1 and 2, a demon seduces men in order to graft their skin over her original demonic form. The seven-year-old boy in the story is neither alien nor demon, but his story brings these sci-fi films easily to mind.
In Bochum, Germany, a small boy throws himself down a playground slide, landing triumphantly at the bottom. To the relief of his father, he is not bleeding. For seven-year-old Hassan, this is a very big day.
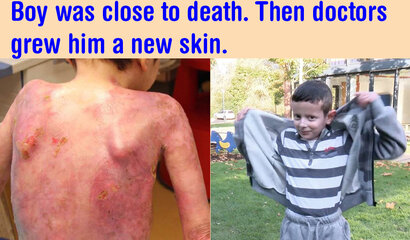
Two years earlier, in June 2015, Hassan was admitted to the burns unit of Ruhr University hospital in Bochum. Covered in blisters, 80 per cent of his skin was open and bleeding. It was one of the most severe cases that doctors had experienced. Attached permanently to a morphine drip, his pain was so extreme that doctors expected him to die.
In November 2016, Tobias Hirsch, a plastic surgeon at the university hospital, successfully transplanted new skin to almost the entirety of Hassan's body. All the skin was grown in a laboratory by Michele de Luca, a world-renowned expert in epithelial stem cell biology.
"As I saw the results after the first operation it was like a miracle to me," Hirsch says. "There really was new, solid skin". In October 2015, and to the surprise of all the surgeons working on the case, the first transplantation to Hassan's four limbs was a success. For the first time doctors were able to prove that transgenic stem cells could regenerate an entire tissue. In simple terms, they realised it was possible not just to grow skin, but also for the skin to thrive when transplanted.
Hassan had a severe case of a rare genetic skin disease known as junctional epidermolysis bullosa (JEB). His skin was so fragile he was covered in wounds, blisters and recurrent infections that were largely untreatable. The entirety of his back and legs were covered in open sores.
Hassan had been transferred to the specialist burns centre as a last resort when his condition began to rapidly deteriorate and the condition spread across his whole body. "When we got him in our burns centre he was in a septic state, so we had a lot of problems from the first day keeping this kid alive," Hirsch says.
First, Hirsch treated Hassan with antibiotics and an aggressive nutrition schedule, but with no success. The team of doctors treating him grew more concerned. Hirsch addressed various experts in the field, in Germany, Switzerland and the United States, in order to find the right experimental treatment. "We tried to transplant the patient some skin that we took from his father," says Hirsch, but this was rejected. "After nearly two months we were sure we could do nothing for this kid and that he would die."
That was when Hassan's father found the work of Michele de Luca. "We studied the literature again and approached Doctor Michele de Luca and his team."

The images on the left show Hassan's massive skin loss before treatment.
The images to the right demonstrate his recovery and the elasticity of his skin
Credit: Hirsch et. al, Nature Research
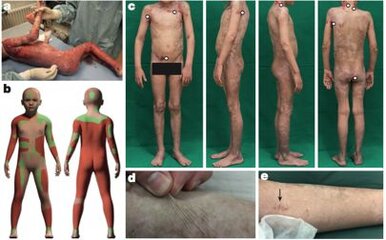
Michele de Luca has worked in epithelial stem cell biology for 20 years. De Luca told the Bochum team that he could grow Hassan enough skin inside his lab to replace the 80 per cent of that he had lost. Scientists had never tried to transplant this magnitude of lab grown skin on to a human. De Luca agreed to take a four-centimetre-square biopsy from an unaffected part of Hassan's skin and began to grow the corrected cell population at his Centre for Regenerative Medicine in Bologna, Italy.
The process took four weeks. "The basic technology is very similar to burns treatment that we have been using in the past," de Luca says. "In other words, you take a biopsy, you isolate the polygenic cells, then the difference is that you genetically modify those cells and make genetic corrections to introduce the new copy of the gene, in this case LAMB3 which is needed to restore the original machinery in the skin." The sets of cells are grown in large numbers, half of them being used to prepare the culture grafts of different sizes. The grafts ranged from 50-100 centimetres in size. After a month, De Luca was ready to transport the graft to the hospital in Bochum, so they could be applied to Hassan.
Transplanting the skin was extremely meticulous and risky. "We first divided the wounds and open areas thoroughly in order to achieve a clean wound bath with a good cleaning situation," Hirsch says. It is essential to get rid of all the bacteria that is placed on the chronic wounds. Then Hirsch applied the grafts provided by De Luca. "What is quite challenging is to make sure the grafts attach properly to the wounds because in this patient you can't use any plastic strips to fix them." The transplanted skin naturally adheres to the wound bed with the help of the right medication. If kept in place, they fully engraft to the body in a few weeks. The team felt confident to proceed, completing the transplantation to the rest of his body the following month. The results of the work were published in the journal Nature.
Crucially for Hassan, this treatment set his recovery apart from previous skin transplant procedures. Usually, due to the severity of the skin damage, burn victims are unable to grow hair on their transplanted skin or develop functioning sweat glands. For these patients, an ointment must be applied to the transplanted skin twice a day for the rest of their life. "We found here that the appendages in the skin didn't need any ointment for the child, we saw hair regrowth and no further need for ointment which is fantastic," Hirsch says. Hassan's parents were overwhelmed. "They told me this was like a dream," Hirsch says.
Eight months after his surgery, Hassan was discharged from hospital. Now, Hassan plays football, goes to school and enjoys life with his siblings. For the past two years, De Luca and Hirsch have kept a close eye on him. Now, they are finally confident his recovery has been successful. "To move from constantly being on morphine to playing football is obviously quite an improvement," Hirsch says. "For me, as Hassan`s surgeon, it is a great success to save his life and bring him back to an almost normal daily life," he adds.
But the impact of this groundbreaking procedure goes beyond a single case. "For the first time we are able to show that an insufficient solid organ has been successfully replaced by genetically-modified stem cells," Hirsch explains. "I worked during my whole research career on gene transfer in skin and wounds. However, we have never applied such a treatment to a patient before."
Michele De Luca is now undertaking trials on two more patients with similar genetic diseases. "We might have problems that we did not encounter before," he says. "This is going to be discovered only after a phase one clinical trial. We are running other clinical trails at the moment." But for Hassan, very simply, it means a new life.
SOURCE
In Under the Skin, Scarlett Johanssen plays an alien who seduces men in order to graft their skin to her black mass of a body. In Painted Skin 1 and 2, a demon seduces men in order to graft their skin over her original demonic form. The seven-year-old boy in the story is neither alien nor demon, but his story brings these sci-fi films easily to mind.
In Bochum, Germany, a small boy throws himself down a playground slide, landing triumphantly at the bottom. To the relief of his father, he is not bleeding. For seven-year-old Hassan, this is a very big day.
Two years earlier, in June 2015, Hassan was admitted to the burns unit of Ruhr University hospital in Bochum. Covered in blisters, 80 per cent of his skin was open and bleeding. It was one of the most severe cases that doctors had experienced. Attached permanently to a morphine drip, his pain was so extreme that doctors expected him to die.
In November 2016, Tobias Hirsch, a plastic surgeon at the university hospital, successfully transplanted new skin to almost the entirety of Hassan's body. All the skin was grown in a laboratory by Michele de Luca, a world-renowned expert in epithelial stem cell biology.
"As I saw the results after the first operation it was like a miracle to me," Hirsch says. "There really was new, solid skin". In October 2015, and to the surprise of all the surgeons working on the case, the first transplantation to Hassan's four limbs was a success. For the first time doctors were able to prove that transgenic stem cells could regenerate an entire tissue. In simple terms, they realised it was possible not just to grow skin, but also for the skin to thrive when transplanted.
Hassan had a severe case of a rare genetic skin disease known as junctional epidermolysis bullosa (JEB). His skin was so fragile he was covered in wounds, blisters and recurrent infections that were largely untreatable. The entirety of his back and legs were covered in open sores.
Hassan had been transferred to the specialist burns centre as a last resort when his condition began to rapidly deteriorate and the condition spread across his whole body. "When we got him in our burns centre he was in a septic state, so we had a lot of problems from the first day keeping this kid alive," Hirsch says.
First, Hirsch treated Hassan with antibiotics and an aggressive nutrition schedule, but with no success. The team of doctors treating him grew more concerned. Hirsch addressed various experts in the field, in Germany, Switzerland and the United States, in order to find the right experimental treatment. "We tried to transplant the patient some skin that we took from his father," says Hirsch, but this was rejected. "After nearly two months we were sure we could do nothing for this kid and that he would die."
That was when Hassan's father found the work of Michele de Luca. "We studied the literature again and approached Doctor Michele de Luca and his team."
The images on the left show Hassan's massive skin loss before treatment.
The images to the right demonstrate his recovery and the elasticity of his skin
Credit: Hirsch et. al, Nature Research
Michele de Luca has worked in epithelial stem cell biology for 20 years. De Luca told the Bochum team that he could grow Hassan enough skin inside his lab to replace the 80 per cent of that he had lost. Scientists had never tried to transplant this magnitude of lab grown skin on to a human. De Luca agreed to take a four-centimetre-square biopsy from an unaffected part of Hassan's skin and began to grow the corrected cell population at his Centre for Regenerative Medicine in Bologna, Italy.
The process took four weeks. "The basic technology is very similar to burns treatment that we have been using in the past," de Luca says. "In other words, you take a biopsy, you isolate the polygenic cells, then the difference is that you genetically modify those cells and make genetic corrections to introduce the new copy of the gene, in this case LAMB3 which is needed to restore the original machinery in the skin." The sets of cells are grown in large numbers, half of them being used to prepare the culture grafts of different sizes. The grafts ranged from 50-100 centimetres in size. After a month, De Luca was ready to transport the graft to the hospital in Bochum, so they could be applied to Hassan.
Transplanting the skin was extremely meticulous and risky. "We first divided the wounds and open areas thoroughly in order to achieve a clean wound bath with a good cleaning situation," Hirsch says. It is essential to get rid of all the bacteria that is placed on the chronic wounds. Then Hirsch applied the grafts provided by De Luca. "What is quite challenging is to make sure the grafts attach properly to the wounds because in this patient you can't use any plastic strips to fix them." The transplanted skin naturally adheres to the wound bed with the help of the right medication. If kept in place, they fully engraft to the body in a few weeks. The team felt confident to proceed, completing the transplantation to the rest of his body the following month. The results of the work were published in the journal Nature.
Crucially for Hassan, this treatment set his recovery apart from previous skin transplant procedures. Usually, due to the severity of the skin damage, burn victims are unable to grow hair on their transplanted skin or develop functioning sweat glands. For these patients, an ointment must be applied to the transplanted skin twice a day for the rest of their life. "We found here that the appendages in the skin didn't need any ointment for the child, we saw hair regrowth and no further need for ointment which is fantastic," Hirsch says. Hassan's parents were overwhelmed. "They told me this was like a dream," Hirsch says.
Eight months after his surgery, Hassan was discharged from hospital. Now, Hassan plays football, goes to school and enjoys life with his siblings. For the past two years, De Luca and Hirsch have kept a close eye on him. Now, they are finally confident his recovery has been successful. "To move from constantly being on morphine to playing football is obviously quite an improvement," Hirsch says. "For me, as Hassan`s surgeon, it is a great success to save his life and bring him back to an almost normal daily life," he adds.
But the impact of this groundbreaking procedure goes beyond a single case. "For the first time we are able to show that an insufficient solid organ has been successfully replaced by genetically-modified stem cells," Hirsch explains. "I worked during my whole research career on gene transfer in skin and wounds. However, we have never applied such a treatment to a patient before."
Michele De Luca is now undertaking trials on two more patients with similar genetic diseases. "We might have problems that we did not encounter before," he says. "This is going to be discovered only after a phase one clinical trial. We are running other clinical trails at the moment." But for Hassan, very simply, it means a new life.
SOURCE
Attachments
Last edited: