Symbianize Forum
Most of our features and services are available only to members, so we encourage you to login or register a new account. Registration is free, fast and simple. You only need to provide a valid email. Being a member you'll gain access to all member forums and features, post a message to ask question or provide answer, and share or find resources related to mobile phones, tablets, computers, game consoles, and multimedia.
All that and more, so what are you waiting for, click the register button and join us now! Ito ang website na ginawa ng pinoy para sa pinoy!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
GENETICS: Unlocking Humanity's Past and Future
- Thread starter Stormer0628
- Start date
- Replies 103
- Views 12,499
More options
Who Replied?- Messages
- 2,145
- Reaction score
- 5
- Points
- 28
- Thread Starter
- #62
Tiny tardigrades—also known as water bears and moss piglets—have many strange properties, including being able to withstand huge amounts of radiation, temperatures ranging from 150°C (302°F) to near absolute zero, and pressures six times greater than in the deepest ocean trenches.
They can have sex in space, be brought to life after being frozen solid for decades, and they look like this. Yep, tardigrades are impossibly weird and indestructible, and now scientists have figured out how to impart their unique ability to form living glass to other organisms.
If you're not familiar with this particular tardigrade superpower, for years, scientists have been trying to figure out how these microscopic creatures can survive years of extreme desiccation and near—100 percent water loss.
"They can remain like that in a dry state for years, even decades, and when you put them back in water, they revive within hours," Thomas Boothby from the University of North Carolina told The New York Times.
"They are running around again, they are eating, they are reproducing like nothing happened."
It was assumed that their survival relied on a sugar called trehalose, which is what brine shrimps—or sea monkeys—use to preserve their cells during desiccation.
But research has shown that trehalose levels are much lower in dried-out tardigrades than they are in brine shrimp, so that couldn't be the solution.
Turns out, what Boothby and his colleagues discovered in 2015 ended up being weirder than anyone had imagined—tardigrades produce a special type of 'bioglass' that holds their essential proteins and molecules in a suspended state until they're rehydrated back to life.
So water bears coat themselves in living glass when they're dried out, and plain old water will melt their proteins back to normal and snap them out of suspended animation.
Since identifying the proteins responsible for the production of this bioglass, called intrinsically disordered proteins (IDPs), Boothby and his team have figured out that they could engineer other creatures to carry these proteins and survive desiccation themselves.
As their name suggests, intrinsically disordered proteins are shapeless and highly flexible under normal conditions, but when extreme drying occurs, their production goes into hyperdrive, and they rearrange themselves into solid biological glasses.
These newly formed IDP glass structures target specific proteins, molecules, and other essential cell parts when the tardigrade starts losing water, and enclose them in stiff, protective envelopes so they don't fall apart during the desiccation process.
When the tardigrade is exposed to water, the glass melts and the IDPs return to their floppy, random state.
Weirdly enough, these proteins are essential for tardigrades to survive desiccation, but when Boothby and his team engineered water bears with low levels of IDPs, they did not affect their resilience against other stresses such as freezing.
When the team inserted these genes into living yeast and bacteria, they found that the TDPs protected them from extreme desiccation just like the tardigrades.
"TDPs are required to protect the tardigrades themselves from desiccation, but they also increase desiccation tolerance when put into bacteria and yeast," Boothby told George Dvorsky at Gizmodo.
"Amazingly, TDPs are sufficient even in a test tube to protect purified biological material like desiccation-sensitive enzymes. The glassy solids that they form are thought to coat desiccation sensitive molecules and physically prevent them from breaking apart, unfolding, or fusing."
While we've been hearing about this work for the past couple of years, we couldn't get too excited about the results because they had not been independently verified through peer-review.
The team's results have now officially been published, which means we have the proof-of-concept in hand to go and make this tardigrade superpower work for us.
And when I say "us", it's actually a possibility, because while yeast and bacteria aren't the most exciting organisms in the world, there are hints that TDPs could work in larger, more complex creatures too.
When the team decided to express the gene that controls IDP production in specially engineered human epithelial cells (HeLa), they found that it produced bioglass upon desiccation.
And a separate experiment last year also found that the protein that protects tardigrades from crazy amounts of radiation can be transferred to cultures of human cells, which really does send the mind boggling.
Other potential applications include developing crops that can survive severe, long-lasting droughts, or even medications that can finally be stored at room temperature instead of having to be constantly chilled—something that makes supply in remote and developing communities extremely difficult.
"Being able to stabilise sensitive pharmaceuticals in a dry state is very important to me personally," says Boothby.
"I grew up in Africa, where lack of refrigeration in remote areas is a huge problem. These real-world applications are one of the things that led me to study tardigrades."
The paper has been published in Molecular Cell.
SOURCE
- - - Updated - - -
The Tardigrade: practically invinsible indestructible water bears
Attachments
- Messages
- 2,145
- Reaction score
- 5
- Points
- 28
- Thread Starter
- #63
If you want to change the yeast in beer or splice genes in an organism, this is the intro class for you.
It's never been easier to learn how to code using online resources, and that's not limited to computer programming. You know what else is code? DNA, which scientists have been modifying in bacteria and embryos with increasing success over the years. Researchers from the Centre Recherches Interdisciplinaires in Paris, France have launched Synthetic Biology One, a website offering free courses to teach anyone how to change genetic code.
Yes, these online classes train you to genetically modify organisms, as it were. But they aim to give you the tools for less controversial DNA alteration, like create specific types of bread, cheese, beer, or yogurt -- basically taking millennia-old food-making methods and throwing in some mad scientist tinkering. As the site states, graduates should exit the course with the ability to "read, write and create custom DNA sequences."
That means getting your feet wet with a little biology, math and computer science at the college undergraduate level, with instruction assuming you've spent a year studying these fields at a university -- though anyone eager enough to try shouldn't be dissuaded. Alongside learning how to use tools "used in synbio labs around the world," the course introduces the kinds of ethics questions that will come to define how researchers and industries use synthetic biology, which is still a nascent field. To that end, the class is deliberately aimed at teachers, students, journalists and policymakers -- basically anyone curious enough to dip their toes in a cool new field of applied science.
So far, Synthetic Biology One has four "courses," which function as instructional units built around a specific practical project. All told, the final program should contain enough work and instruction to constitute about a semester's-worth of education. You won't get any sort of certificate or college credit, at least for now, nor is the coursework peer-reviewed and industry-approved. But like many MOOCs, free access to intro-level cutting-edge science is a fantastic resource to novices who aren't ready to invest in college classes.
Source: Synthetic Biology One
Attachments
- Messages
- 2,145
- Reaction score
- 5
- Points
- 28
- Thread Starter
- #64
A growing body of evidence continues to reveal that many diseases—from cancer, autism, Alzheimer's disease, bipolar disorder, to schizophrenia, and many others are ultimately linked with the overall health, balance, and diversity of one's gut bacteria or microbiome. Gut bacteria are also found to affect how we perceive the world around us—from our immediate family, friends, society at large, as well as events—and our overall mood and mental health. These are all astounding developments, and these also point to a profound realization: that these gut bacteria, which are also found to have complex communication tools to identify friends from enemies, amount to a second brain within our own bodies. Creepy?
The human brain is made up of billions of neurons that are constantly firing electrical signals back and forth to tell us what to do, what to think, and how to feel.
But your body actually has a second brain that controls you much more than you might realise—and most people have no idea it exists.
As this episode of AsapSCIENCE explains, one of the main ways our brain communicates with the rest of our body is via the vagus nerve, which passes messages to the vocal chords, heart, lungs, and the digestive tract.
But researchers have also discovered that within the enteric nervous system—the extensive mesh-like network of neurons that controls your digestive tract - the messages are going the other way, too.
In fact, 80 to 90 percent of the nerve fibers in the enteric nervous system are going from the gut to the brain. And when the vagus nerve is cut, the enteric system doesn't need the brain at all.
In other words, your digestive system is your second brain, and it controls you far more than you realize.
If you're thinking that just because the gut can pass messages back to the brain doesn't mean it's in control, then consider how, it turns out, our digestive system also influences our choices on a daily basis.
This is most likely because back when our ancestors were living as hunter gatherers, some of the most important life-or-death choices they'd have to make were based on food: would eating a berry provide enough energy to get through the day? Would it be poisonous?
Because of this, from an evolutionary perspective, it makes sense to have a direct line of communication between the gut and the brain.
But even today, it's incredibly influential.
Not only has research shown that our gut bacteria can manipulate our food cravings and behavior in order to ensure their own survival (you can blame them on your junk food obsession), but the colonies in our digestive system also affect our mood.
Studies suggest that people with healthy and diverse gut microbiomes are less likely to be depressed or anxious.
And, in mice, researchers have shown that those that grow up in sterile environments—where no bacteria colonize their guts—display social traits similar to those in humans on the autism spectrum. When these mice were fed probiotics, their symptoms were alleviated.
This kind of effect has been seen in early studies in humans too, leading many scientists to believe that one of the primary functions of gut bacteria is actually to promote social behaviors and ensure the survival of the species through reproduction—but we'll let the AsapSCIENCE video above explain that.
In some ways, our second brain is even more influential than our logical thought. And—great—now we can't stop second-guessing which brain is making us do everything. These days, knowing what our bodies do without our own conscious knowledge is turning out to be truly creepy.
SOURCE
Attachments
- Messages
- 2,145
- Reaction score
- 5
- Points
- 28
- Thread Starter
- #65
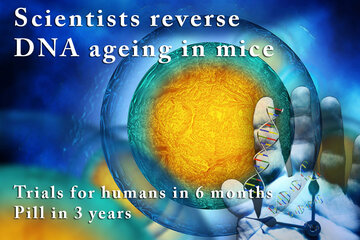
Researchers have identified a cellular mechanism that allows them to reverse ageing in mouse DNA and protect it from future damage.
They've shown that by giving a particular compound to older mice, they can activate the DNA repair process and not only protect against future damage, but repair the existing effects of ageing. And they're ready to start testing in humans within six months.
"The cells of the old mice were indistinguishable from the young mice, after just one week of treatment," said lead researcher David Sinclair from the University of New South Wales (UNSW) in Australia and the Harvard Medical School in Boston.
"This is the closest we are to a safe and effective anti-ageing drug that's perhaps only three to five years away from being on the market if the trials go well."
Sinclair and his team made headlines back in 2013 when they found that the cells of younger mice contained more of a compound called nicotinaminde adenine dinucleotide, or NAD+, than their older counterparts.
Not only that, but when they gave the older mice more NAD+, they started to look younger, too.
It was a big deal at the time, but one of the tricky things about medicine is that in order to show that something could work as a potential treatment, you need to first understand how it's acting in the body.
And although the researchers knew NAD+ was having an impressive effect, they couldn't say for sure how it was doing it.
Now, Sinclair and his team have released a new study, where they outline in detail the mechanism through which NAD+ protects DNA from the damage of ageing and radiation in mice.
So how does it work? When we're born, all of our cells have the ability to repair DNA damage, which we experience constantly through random mutations when our cells divide, or whenever we go out in the sun.
But as we get older, our ability to patch up this damage declines, and our cells being to age.
What the researchers have now shown in this latest study is that a lot of this damage comes down to a DNA-repair compound called PARP1.
When there's a lot of NAD+ in a cell, PARP1 does its job and keeps our DNA healthy. But when NAD+ drops naturally with age, PARP1 starts to decline, and damage builds up.
To see whether they could take advantage of this cellular mechanism, Sinclair and his team developed a drug that contains the precursor to NAD+, known as NMN, or nicotinamide mononucleotide.
In mice, boosting older mice with NMN was enough to kick DNA repair into action and even reverse existing DNA damage.
They now plan to trial a similar drug in humans before the end of the year—and not just for anti-ageing purposes, but also to protect against DNA damage of any kind.
In fact, the team is collaborating with NASA to see if NMN could help protect its astronauts against harsh space radiation on their four-year journey to Mars, during which it's predicted that 5 percent of the astronauts' cells would die, and their chances of cancer would approach 100 percent.
The drug could also be useful for groups that are particular vulnerable to the effects of radiation, such as frequent fliers and those who undergo frequent CT scans or X-rays.
"The idea is to protect the body from radiation exposure here on earth, either naturally occurring or doctor-inflicted," Sinclair told Time.
"If I were going to have an X-ray or a CT scan, I would take NMN beforehand."
Survivors or childhood cancer could also benefit—right now, 96 percent go on to suffer a chronic illness by age 45, including cardiovascular disease, Alzheimer's, or cancers unrelated to their original cancer.
"All of this adds up to the fact they have accelerated ageing, which is devastating," said one of the researchers, Lindsay Wu. "It would be great to do something about that, and we believe we can with this molecule."
Before we get too excited, we need to keep in mind that many, many studies in mice are not replicated in humans.
So until the results of these early clinical trials in people begin to trickle in, there's no promise that NMN will help protect human DNA.
But understanding this mechanism of DNA ageing is a big step towards better understanding how to keep our cells healthier for longer, and that's pretty exciting.
The research has been published in Science.
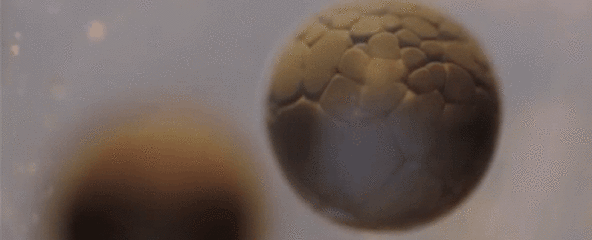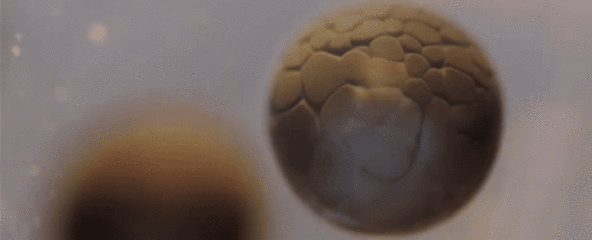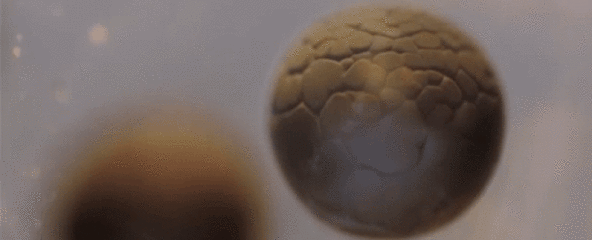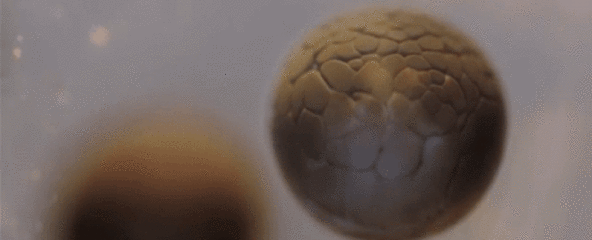
Spectacular Time-Lapse of Cell Division

If you've ever wondered what cell division actually looks like, this incredible time-lapse by francischeefilms on YouTube gives you the best view we've ever seen, showing a real-life tadpole egg dividing from four cells into several million in the space of just 20 seconds:
Of course, that's lightning speed compared to how long it actually takes—according to Adam Clark Estes at Gizmodo, the time-lapse has sped up 33 hours of painstaking division into mere seconds for our viewing pleasure.
The species you see developing here is Rana temporaria, the common frog, which lays 1,000 to 2,000 eggs at a time in shallow, fresh water ponds.
According to the team behind the footage, they had to build their own equipment to film it like this, and had to devise a way to get the lighting and microscope set-up just right.
"The whole microscope sits on anti-vibration table. t doesn't matter too much what microscope people use to perform this," francischeefilms describe on their YouTube page.
"There are countless other variables involved in performing this tricky shot, such as: the ambient temperature during shooting; the time at which the eggs were collected; the handling skills of the operator; the type of water used; lenses; quality of camera etc."
Check out the footage above, marvel at life itself, and then let SciShow tell you how it all ends—on a cellular level:
Attachments
- Messages
- 2,145
- Reaction score
- 5
- Points
- 28
- Thread Starter
- #67
Daghang salamat sa tanan
Welcome. Balik-balik lang kay pirmi ko man ni sya ni-update.
- - - Updated - - -
Here's a video for the previous post topic: scientists reverse aging in mice, human trials to start in 6 months:
- - - Updated - - -
and another one....
- - - Updated - - -
Straight from the mouth of Sinclair, the chief scientist of the research himself, on how they made it work....
Last edited:
- Messages
- 2,145
- Reaction score
- 5
- Points
- 28
- Thread Starter
- #68
Here's a study that should be either satisfying or disappointing for some people depending on their chosen lifestyle.
Almost two-thirds of cancer mutations are caused by random DNA-copying errors during cell division and are impossible for us to avoid, regardless of lifestyle and the genes we inherit from our parents, according to new research.
The findings—which estimate that 66 percent of cancer mutations are effectively bad luck that we can't do anything about—support the conclusions of a controversial paper released in 2015 by the same researchers, which came under fire for appearing to suggest that there was nothing we could do to prevent various cancers.
This time around, the team from Johns Hopkins University are at pains to emphasize that their findings don't contradict what we know about cancer prevention—nor detract from the importance of environment and heredity in terms of causing cancer.
"It is well-known that we must avoid environmental factors such as smoking to decrease our risk of getting cancer. But it is not as well-known that each time a normal cell divides and copies its DNA to produce two new cells, it makes multiple mistakes," says biostatistician Cristian Tomasetti.
"These copying mistakes are a potent source of cancer mutations that historically have been scientifically undervalued, and this new work provides the first estimate of the fraction of mutations caused by these mistakes."
When Tomasetti and geneticist Bert Vogelstein last examined the role of these random mutations driving cancer growth, they were criticized for only examining US heath data—and for not providing results on breast or prostate cancer.
In the new study, to broaden the scope of their original findings, they examined medical data from 68 countries on 32 types of cancer—including breast and prostate—to find what proportion of mutations that cause cancer are due to random mistakes made during DNA copying.
The team found that the extent to which random mutations contribute to cancer growth differs between each kind of cancer, but it certainly looks to be a significant factor.
In the case of pancreatic cancer, the researchers say that 77 percent of the mutations that cause tumors are due to random DNA copying mistakes, whereas 18 percent are down to environmental factors, with inherited genes accounting for the remaining 5 percent.
In other cancers—including prostate, brain, and bone cancer—the researchers found that 95 percent of cancer mutations are a result of cell division errors.
For some cancers, though, bad luck appears to play a smaller role.
In lung cancer, 65 percent of mutations are due to environment—such as smoking, or living in a polluted area—whereas DNA copying errors account for the other 35 percent, with heredity playing no role.
Overall, the team estimates that 66 percent of cancer mutations are due to random, unavoidable mistakes made during cell division, with 29 percent being attributable to environment, and 5 percent being inherited.
Despite the controversy that greeted their results last time, the authors say their findings actually complement the scientific consensus on cancer: that approximately 40 percent of cases can be prevented.
"We need to continue to encourage people to avoid environmental agents and lifestyles that increase their risk of developing cancer mutations," says Vogelstein.
"However, many people will still develop cancers due to these random DNA copying errors, and better methods to detect all cancers earlier, while they are still curable, are urgently needed."
Despite the sweeping nature of the new analysis, not everybody is convinced by the new findings.
Some researchers say it's too simplistic to arbitrarily conclude that cancer as a whole can be divvied up neatly into three key categories—random mutations, environment, and heredity—arguing that the interplay between these factors could itself be another contributor.
"We're not saying the only thing that determines the seriousness of the cancer, or its aggressiveness, or its likelihood to cause the patient's death, are these mutations," Vogelstein told Richard Harris at NPR.
"We're simply saying that they are necessary to get the cancer."
If the findings end up being accepted by other cancer researchers, the idea that randomness—in other words, bad luck—is more significant in causing cancer than other contributing factors could amount to what Tomasetti calls "a complete paradigm shift in how we think about cancer and what causes cancer.”
On one hand, it could make it easier for cancer patients and their families to process their diagnoses – especially in cases where conscious lifestyle choices (to not smoke, or to eat healthily) weren't rewarded.
"They need to understand that these cancers would have happened no matter what they did," Vogelstein told media at a press conference on Thursday.
"We don't need to add guilt to an already tragic situation."
The findings could also help steer research to investigate the biological processes behind random mutations that we don't currently understand. While there's nothing we can do to prevent natural DNA copying mistakes made at the cellular level today, that might not always be the case.
"Something we don't consider a modifiable risk factor today might look modifiable in the future," geneticist Paul Meltzer from the US National Cancer Institute's Center for Cancer Research, who wasn't involved with the study, told the Los Angeles Times.
"What we call the 'bad luck' in the gene replication, we may have ways to reduce that in the future."
The findings are reported in Science.
SOURCE
Attachments
- Messages
- 2,145
- Reaction score
- 5
- Points
- 28
- Thread Starter
- #69
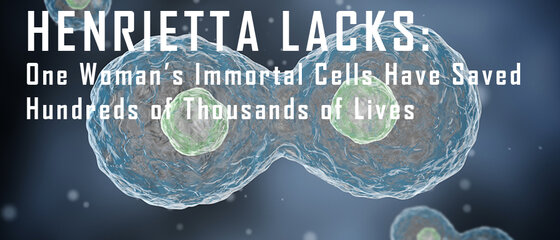
Imagine this: you’re a physician at one of the most renowned medical institutions in the world. A patient comes in with a very aggressive form of cervical cancer and you collect her cells to study them for her particular case. But while studying her cells, you notice something odd. After a certain number of cell divisions, they don’t die like other cells in the research lab. In fact, they don’t die at all.
It’s the year 1950 and it’s years before any regulations on harvesting cells from patients are mandated. In fact, there is no custom of asking permission for cell collection for research. With that said, you think about all the potential these “immortal cells” may have. You have never seen cells quite like them before in your life, and you know for a fact that your colleagues and other researchers around the world have not either.
What do you do?
To George Otto Gey, it was simple: collect the cells and propagate them for future research. His patient was a 31-year-old woman named Henrietta Lacks.
THE BIRTH OF A MEDICAL REVOLUTION
Henrietta Lacks’s cancer cells were quickly dubbed “HeLa cells” in the scientific community. Even 60 years after their collection, HeLa cells are still the most commonly-used cell line around the world, having been produced billions and billions of times now. This is because HeLa cells were the first cells to survive in vitro (in a test tube) and provided the foundation for some of the most remarkable discoveries in modern medicine.
If you have ever had the polio vaccine, you can thank Henrietta Lacks for her cells. From 1840 to 1950, Poliomyelitis was a lethal global epidemic that not even President Franklin D. Roosevelt was spared from. He declared a war on the disease. The vaccine developed by Jonas Salk in 1952 was only possible because HeLa cells were able to survive in vitro. The HeLa cells were easy to infect and study, and therefore provided the perfect subject for Dr. Salk to utilize in his research. With only 403 cases in 2014, polio has been on the run. The vaccine has prevented 650,000 deaths and 13 million cases of paralysis since 1988. All of this would not have been possible if it weren’t for Henrietta Lacks’s immortal cell line.
Henrietta Lacks’s cells were the foundation of opening the field of virology in the first place. But that’s not all: her contribution to modern medicine moves past just vaccines. Her cells have allowed us to study cancer, cell growth in space, HIV, the human genome, Tuberculosis, HPV, Parkinson’s disease, and even cosmetics.
Henrietta Lacks’s contribution to modern medicine is clear—without her cells, who knows where we would be today? While medical progress is often thought of as inevitable, it’s by chance events like the discovery of Lacks’s cells that the rate of progress is decided. Her cells were the catalyst to many medical advances that we have today.
For more information:
SOURCE
Attachments
- Messages
- 2,145
- Reaction score
- 5
- Points
- 28
- Thread Starter
- #70
Octopuses have three hearts, parrot-like beaks, venomous bites, and eight semi-autonomous arms that can taste the world. They squirt ink, contort through the tiniest of spaces, and melt into the world by changing both color and texture. They are incredibly intelligent, capable of wielding tools, solving problems, and sabotaging equipment. As Sy Montgomery once wrote, “no sci-fi alien is so startlingly strange” as an octopus. But their disarming otherness doesn’t end with their bodies. Their genes are also really weird.
A team of scientists led by Joshua Rosenthal at the Marine Biological Laboratory and Eli Eisenberg at Tel Aviv University have shown that octopuses and their relatives—the cephalopods—practice a type of genetic alteration called RNA editing that’s very rare in the rest of the animal kingdom. They use it to fine-tune the information encoded by their genes without altering the genes themselves. And they do so extensively, to a far greater degree than any other animal group.
“They presented this work at a recent conference, and it was a big surprise to everyone,” says Kazuko Nishikura from the Wistar Institute. “I study RNA editing in mice and humans, where it’s very restricted. The situation is very different here. I wonder if it has to do with their extremely developed brains.”
It certainly seems that way. Rosenthal and Eisenberg found that RNA editing is especially rife in the neurons of cephalopods. They use it to recode genes that are important for their nervous systems—the genes that, as Rosenthal says, “make a nerve cell a nerve cell.” And only the intelligent coleoid cephalopods—octopuses, squid, and cuttlefish—do so. The relatively dumber nautiluses do not. “Humans don’t have this. Monkeys don’t. Nothing has this except the coleoids,” says Rosenthal.
It’s impossible to say if their prolific use of RNA editing is responsible for their alien intellect, but “that would definitely be my guess,” says Noa Liscovitch-Brauer, a member of Rosenthal’s team who spearheaded the new study. “It makes for a very compelling hypothesis in my eyes.”
Here’s how RNA editing works. Genes encode instructions in the form of DNA—in the sequence of four building blocks represented by the letters A, C, G, and T. For those instructions to be used, the DNA must first be transcribed into a similar molecule called RNA, which contains roughly the same building blocks. The RNA is then translated and used to build proteins—the molecular machines that carry out all the important jobs inside our cells. So DNA stores information, RNA carries it, and proteins are the result of it.
That’s the simple version. But the RNA often gets altered before it’s used to make proteins. Some of the changes are big—large sections are cut out, and the remaining pieces are glued back together. Other changes are small—sometimes, a single A gets converted into an I (which is functionally equivalent to a G). That’s RNA editing. It’s performed by a group of enzymes called ADARs, which recognize specific sequences of RNA and makes those A-to-I changes.
But to what end? RNA editing is still mysterious, and its purpose unclear. Technically, an animal could use it to change the nature of its proteins without altering the underlying DNA instructions. But in practice, this kind of recoding is extremely rare. Only about 3 percent of human genes are ever edited in this way, and the changes are usually restricted to the parts of RNA that are cut out and discarded. To the extent that it happens, it doesn’t seem to be adaptive.
In cephalopods, it’s a different story. Back in 2015, Rosenthal and Eisenberg discovered that RNA editing has gone wild in the longfin inshore squid—a foot-long animal that’s commonly used in neuroscience research. While a typical mammal edits its RNA at just a few hundred sites, the squid was making some 57,000 such edits. These changes weren’t happening in discarded sections of RNA, but in the ones that actually go towards building proteins—the so-called coding regions. They were ten times more common in the squid’s neurons than in its other tissues, and they disproportionately affected proteins involved in its nervous system.
Having been surprised by one cephalopod, the team decided to study others. Liscovitch-Brauer focused on the common cuttlefish, common octopus, and two-spot octopus. All of these showed signs of extensive RNA editing with between 80,000 to 130,000 editing sites each. By contrast, the nautilus—a ancient cephalopod known for its hard, spiral shell—only had 1,000 such sites.
This distinction is crucial. The nautiluses belong to the earliest lineage of cephalopods, which diverged from the others between 350 and 480 million years ago. They’ve stayed much the same ever since. They have simple brains and unremarkable behavior, and they leave their RNA largely unedited. Meanwhile, the other cephalopods—the coleoids—came to use RNA editing extensively, and while evolving complex brains and extraordinary behavior. Coincidence?
Liscovitch-Brauer also found that around 1,000 of the edited locations were shared between the coleoid species—far more than the 25 or so sites that are shared between humans are other mammals. These sites have been preserved over hundreds of millions of years of evolution. “That’s pretty convincing evidence that these edits are integrated into the wiring of these genomes, and that disrupting this network of edits would be harmful in some way,” says Daniel Rokhsar from the University of California, Berkeley, who was not involved in the study.
Indeed, the edits seem to be so important that cephalopods have gone to unusual lengths to preserve them. To find and edit a particular RNA letter, ADAR enzymes rely on all the surrounding letters for clues. It’s like finding this single ‘A’ by looking for all the words in this paragraph.
This means that editing sites effectively cordon off a large chunk of a cephalopod’s genome—between 23 and 41 percent by Rosenthal’s reckoning. Those sites must remain largely unchanged, or the editing enzymes won’t be able to find their targets. And this means that octopus and squid genomes evolve at a slower pace than those of other animals. They may be icons of flexibility and change, but their genomes are rigid and stagnant.
Rosenthal thinks that they pay for this sacrifice with a different kind of flexibility. By changing their RNA rather than their DNA, they might be more effective at adapting to challenges on the fly. From the same gene, they could produce proteins that, say, work better in hot temperatures or cold ones. And such changes would be temporary—the creatures could turn them on or off depending on the circumstance.
Rosenthal wonders if they could learn or encode experiences in this way. “I’m working a lot on the squid ADAR enzymes and their distribution between cells,” he says. “It’s mind-blowing how variable they are. One neuron will have high levels but its neighbor will have nothing.”
“This study suggests that RNA editing and recoding is important in the function of the largest invertebrate brains,” says Carrie Albertin from the University of Chicago, who helped to sequence the first cephalopod genome. “By comparing vertebrate and cephalopod brains, we can understand how large nervous systems are put together.”
“It’s a really interesting phenomenon, but it’s unclear why they need so much RNA editing,” says Jianzhi Zhang from the University of Michigan. “It’s not absolutely clear if it has to do with behavior; humans have very complex brains and behaviors and in us, RNA editing is very rare.” The question isn’t just why coleoid cephalopods are unique in embracing RNA editing, but why nothing else has to the same extent.
So far, the team has a lot of correlations—compelling ones, but correlations nonetheless. Rosenthal’s next move is to develop ways of genetically manipulating cephalopods. If he succeeds, he could disable their ADAR enzymes, stop them from editing their RNA, and see what happens.
SOURCE
Attachments
- Messages
- 2,145
- Reaction score
- 5
- Points
- 28
- Thread Starter
- #71
An innovative study in the University of Wisconsin-Madison could soon put an end to drug-resistant bacteria by using an edible version of CRISPR. This probiotic could target specific bacteria, making it more effective than antibiotics.
TARGETED AND EDIBLE
As antibiotic resistance continues to grow, scientists are desperately trying to figure out how to best fight bacterium like Clostridium difficile, which can cause fatal infections in hospitals and nursing homes. C. difficile has been ranked by the U.S. Centers for Disease Control and Prevention (CDC) as a top drug-resistant threat responsible for about 15,000 deaths per year. Several means are being explored to counter the pandemic, the most recent coming from a project funded by the National Institute of Health.
The proposed solution uses CRISPR, the world’s most precise and efficient gene editing technology currently available. Jan-Peter van Pijkeren, a food scientist from the University of Wisconsin-Madison, is creating a probiotic cocktail that patients can swallow as a liquid or pill.
The cocktail of bacteria will include a bacteriophage – a virus that infects bacteria – capable of carrying a customized, false, CRISPR message to C. difficile. This message would cause C. difficile to make lethal cuts to its own DNA.
BETTER THAN ANTIBIOTICS
Currently, this probiotic is still in its early stages, according to van Pijkeren, and is yet to be tested on animals. Luckily, similar studies have proven the effectiveness of bacteriophage-delivered CRISPR in killing bacteria. However, researchers still have some concerns.
“The downside of antibiotics is they are a sledgehammer that depletes and destroys the gut microbial community,” van Pijkeren said to the University of Wisconsin-Madison. “You want to instead use a scalpel in order to specifically eradicate the microbe of interest.”
CRISPR is ideal for this use because such drugs would be very specific to the user. They could kill a single species of germ while leaving good bacterial untouched. In contract, regular antibiotics kill off both good and bad bacteria, leading to resistance. If proven successful, CRISPR could become, not just the world’s most effective gene editing tool, but also the best bacteria-killing technology available. While this is a long way off, it still gives hope to thousands.
“As long as we house patients together in a hospital or in a nursing home and we give a lot of them antibiotics we’re going to have a problem with C. difficile,” says Herbert DuPont, director of the Center for Infectious Diseases at the University of Texas to MIT Technology Review.
SOURCE
Attachments
- Messages
- 2,145
- Reaction score
- 5
- Points
- 28
- Thread Starter
- #72
Although not many people fancy them in the same way people drop their jaws with tardigrades, hydra, or octopuses, naked mole rats (Heterocephalus glaber) have never been the most conventional mammal—the scrotum-looking creatures are resistant to cancer, can survive almost 20 minutes without oxygen, and can barely feel pain.
But new research has revealed that they're even more bizarre than scientists could have ever imagined.
A new study has shown that when these strange creatures are deprived of oxygen, their metabolism behaves more like a plant than a mammal. Seriously, what the hell, nature?
Unlike all other known mammals, when the brain cells of naked mole rats are deprived of oxygen they do not run out of energy and die.
In fact, naked mole rats can survive for five hours at oxygen levels so low a human would die in minutes, and this latest study showed they can endure a ridiculous 18 minutes of total oxygen deprivation by slipping into a sort of vegetative state. Mice without oxygen die after around 20 seconds.
Previous studies have shown that naked mole rats conserve energy by slowing their movements, reducing their breathing rate, and dramatically slowing their pulse. They're the only mammals known to survive suffocation through this type of suspended animation.
But for a long time, scientists were stumped about how naked mole rats were able to survive these conditions on a cellular level without lasting damage—usually the lack of oxygen causes the build up of harmful metabolites and brain cells to die off.
Now, researchers think they have the answer.
Instead of sticking to a glucose-based system, which is dependent on oxygen, when a naked mole rat is deprived of oxygen, it switches its metabolism so that its brain cells start burning fructose for energy instead of glucose.
Fructose can be turned into energy anaerobically—which means it doesn't require the presence of oxygen to be broken down into cellular energy.
Until now, this anaerobic pathway was thought only to be used by plants.
"This is just the latest remarkable discovery about the naked mole-rat—a cold-blooded mammal that lives decades longer than other rodents, rarely gets cancer, and doesn't feel many types of pain," says lead researcher Thomas Park from the University of Illinois at Chicago.
The team showed that the mammals keep metabolizing fructose in this state until oxygen becomes available, keeping their brain cells alive even without oxygen. At which point, they will perk up and begin moving as if nothing happened. Remarkably, there appears to be no lasting damage.
Scientists believe the naked mole rat adapted this way to survive in overcrowded and unventilated burrows—up to 100 naked mole rats are known to sleep together in mounds to keep warm.
"The naked mole rat has simply rearranged some basic building-blocks of metabolism to make it super-tolerant to low oxygen conditions," says Park.
To figure this out, researchers exposed naked mole rats to conditions of low oxygen and found an increased amount of fructose in their bloodstreams.
Other mammals can metabolize fructose in their intestines, but the team showed that, in naked mole rats, fructose was also being funneled into brain cells by fructose pumps that are only found in the intestine of other mammals.
To take things further, when fructose was supplied directly to the brains and hearts of naked mole rats and mice, these organs were able to survive using fructose as energy, even without the presence of oxygen. Under the same conditions, the hearts and brains of mice failed.
Surprisingly, the naked mole rat's heart performed just as well with fructose as with glucose, suggesting it is just as skilled with one type of metabolism as it is with the other.
The study is important not just because it reveals the true biology of these curious creatures. It also raises questions about whether human cells could survive without oxygen.
"Patients who suffer an infarction or stroke experience irreparable damage after just a few minutes of oxygen deprivation," says one of the researchers Gary Lewin from the Max Delbrück Center for Molecular Medicine in the Helmholtz Association.
"Theoretically, very few changes might be needed to adopt this unusual metabolism."
If human cells could rearrange their metabolism in this way, critical organs could be saved when a stroke or infarction occurs.
Naked mole rats have also recently exposed the secrets of how they're so impervious to pain, which scientists think could help them to develop better pain treatments in humans.
Strange foreskin rats, we salute you.
The research has been published in Science.
SOURCE
Attachments
- Messages
- 2,145
- Reaction score
- 5
- Points
- 28
- Thread Starter
- #74
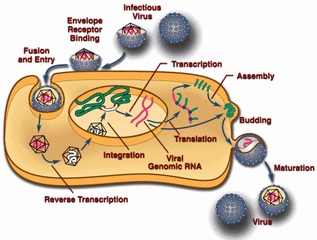
Viruses reproduce by infiltrating living cells and taking over the biological machinery inside. It’s an insidious process that can leave the host with a life-threatening illness, a mild fever, or no ill effects at all. Recent advances in medical science have allowed humans to combat viruses like never before, but a new study from researchers at Rockefeller University shows how our primate ancestors may have waged war on a virus with only the weapon of evolution.
Viruses don’t leave behind fossil evidence, but sometimes their DNA can survive as part of their victims, and that’s where the team went looking in this case. The study focuses on an ancient virus known as HERV-T, which began infecting primates some 32 to 43 million years ago. HERV-T is a retrovirus (just like HIV), which means it carried its genetic material around as RNA. Eukaryotic cells (like ours) are DNA-based, so one of the first things HERV-T did upon gaining access to a cell was turn its RNA into DNA, then it stuffed it into the cell’s DNA to be duplicated.
HERV-T has long since gone extinct, but the researchers were able to find its remains in the genetic material of various primates. The germline cells like fetal cells, sperm progenitors, and eggs that were infected with HERV-T passed the viral genes down over the eons. This is what allowed the team to construct a timeline for the rise and fall of HERV-T, and find out how our ancestors might have killed it.
When HERV-T began popping up in primates around 40 million years ago, it used a protein on host cells called MCT1 to gain access. The virus used a protein called ancHTenv to link up with that protein, like a key in a lock. The team also found a remarkably well-preserved version of that protein hiding in the DNA of primates (including humans), which they’ve named hsaHTenv. It’s not uncommon for organisms to pick up a bit of nucleic acid here and there from viruses, but the nature of this gene suggests some interesting possibilities. The team used the remaining genetic code to reconstruct the ancient virus protein and study its function, which was a scientific first.
The life cycle of a retrovirus.
Scientists postulate that hsaHTenv was captured by our hominid ancestors’ cells around 19 million years ago. This gene was used by cells to produce the “key” protein of the virus independent of the virus itself. This in turn allowed cells to immunize themselves. Basically, hsaHTenv could bind to MCT1 particles in the cell, preventing them from being added to the cell’s membrane. With no MCT1 on the surface, the virus had no way to infect the cell. In the space of a few million years, HERV-T died out as its pool of hosts shrunk.
Now read: How DNA sequencing works
SOURCE
Attachments
- Messages
- 2,145
- Reaction score
- 5
- Points
- 28
- Thread Starter
- #75
First we started reading the human genome. Next we should start writing them. Or should we?
One of the greatest ethical debates in science—manipulating the fundamental building blocks of life—is set to heat up once more.
According to scientists behind an ambitious and controversial plan to write the human genome from the ground up, synthesising DNA and incorporating it into mammalian and even human cells could be as little as four to five years away.
Nearly 200 leading researchers in genetics and bioengineering are expected to attend a meeting in New York City next week, to discuss the next stages of what is now called the Genome Project-write (GP-write) plan: a US$100 million venture to research, engineer, and test living systems of model organisms, including the human genome.
Framed as a follow-up to the pioneering Human Genome Project (HGP)—which culminated in 2003 after 13 years of research that mapped the human genetic code—this project is billed as the logical next step, where scientists will learn how to cost-effectively synthesize plant, animal, and eventually human DNA.
"HGP allowed us to read the genome, but we still don't completely understand it," GP-write coordinator Nancy J. Kelley told Alex Ossola at CNBC.
While those involved are eager to portray the project as an open, international collaboration designed to further our understandings of genome science, GP-write provoked considerable controversy after its first large meet-up a year ago was conducted virtually in secret, with a select group of invite-only experts holding talks behind closed doors.
"Given that human genome synthesis is a technology that can completely redefine the core of what now joins all of humanity together as a species, we argue that discussions of making such capacities real ... should not take place without open and advance consideration of whether it is morally right to proceed," medical ethicist Laurie Zoloth from Northwestern University and synthetic biologist Drew Endy of Stanford University wrote at the time for Cosmos Magazine.
Since then, the researchers behind the initiative have been more candid, announcing details of the project in a paper in Science, as well as releasing a white paper outlining GP-write's timeline and goals.
One of GP-write's lead scientists—geneticist and biochemist Jef Boeke from NYU Langone Medical Centre—says the approach has always been to consult the scientific community at large, to help frame and steer the research as it develops.
"I think articulation of our plan not to start right off synthesising a full human genome tomorrow was helpful. We have a four- to five-year period where there can be plenty of time for debate about the wisdom of that, whether resources should be put in that direction or in another," he told CNBC.
"Whenever it's human, everyone has an opinion and wants their voice to be heard. We want to hear what people have to say."
But while that conversation is taking place, the science is developing regardless.
In March, Boeke shared details on a related project he's involved with, where he oversees hundreds of scientists who are working together to synthesize an artificial yeast genome, which is expected to be complete by the end of 2017.
There might be a large gap between successfully synthesising yeast DNA and creating artificial human DNA from scratch. But the overall goal is to figure out how to synthesize comparatively simple genetic codes (such as microbial and plant DNA), before moving on to the ultimate prize.
"If you do that, you gain a much deeper understanding of how a complicated apparatus goes," says Boeke. "Really, a synthetic genome is an engine for learning new information."
Under its new organizational structure, GP-write is the parent project, which encompasses the core area of Human Genome Project-write (HGP-write), focused on synthesizing human genomes in whole or in part.
In addition to synthesising plant, animal, and human DNA, the primary goal of the project is to lower the cost of engineering genomes.
At present, it's estimated to cost about 10 US cents to synthesize every base pair of nucleobase molecules that make up our DNA—and given humans have about 3 billion of these pairs, that makes for some pretty prohibitively expensive synthesis.
The plan is to reduce this cost by more than 1,000-fold within 10 years.
If that happens, the lower expense involved in synthesising DNA could unlock all kinds of new potential medical treatments—targeting illnesses such as cancer and genetic diseases, helping the body to accept organ transplants, and learning more about immunity to viruses.
Of course, before that can happen, GP-write's organizers need to raise an estimated US$100 million in funding—which will be another of the drivers of next week's get-together.
It's an incredibly exciting undertaking, although there's bound to be more controversy as GP-write marches ahead.
SOURCE
Attachments
- Messages
- 2,145
- Reaction score
- 5
- Points
- 28
- Thread Starter
- #76
The CRISPR gene-editing tool has already shown a lot of potential for helping doctors treat the most stubborn diseases, and now scientists have used it to target the "command center" of cancerous tumors, stopping their growth and boosting survival rates in mice.
In this new study, CRISPR was aimed directly at fusion genes—formed when two genes combine to form a hybrid, resulting in abnormal proteins which often cause cancer or help it to grow.
These fusion genes also have a unique DNA fingerprint, which researchers from the University of Pittsburgh were able to use to hunt down and modify them. Specially engineered viruses were then applied to replace the fusion genes with cancer-killing ones.
"This is the first time that gene-editing has been used to specifically target cancer fusion genes," says lead researcher Jian-Hua Luo. " It is really exciting because it lays the groundwork for what could become a totally new approach to treating cancer."
CRISPR lets scientists effectively cut and paste the DNA in cells to fix problems or make improvements, and it has already been used to boost immune cells in the fight against certain types of cancers.
In this case, the researchers went for one of the causes of growth, demonstrating a new way to tackle the disease.
A type of fusion gene called MAN2A1-FER was targeted—previously identified by the same team as being present in certain types of aggressive cancer in the prostate, liver, lungs, and ovaries.
"Other types of cancer treatments target the foot soldiers of the army," explains Luo. "Our approach is to target the command centre, so there is no chance for the enemy's soldiers to regroup in the battlefield for a comeback."
Once modified, the CRISPR-edited, cancer-killing genes were injected into mice carrying human prostate and liver cancer cells. The tumours reduced in size by up to 30 percent, no secondary growths were noted, and all the mice survived until the end of the eight-week test.
In contrast, in a control group of mice that didn't receive the treatment, the cancer tumours increased nearly 40-fold in size, metastasis or cancer spread was common, and all the animals died before the study ended.
Even better, because fusion genes only occur in cancerous cells, healthy cells are left alone.
This could give the new technique a big advantage over chemotherapy, which has numerous unwanted side effects on healthy parts of the body.
Tackling the fusion genes didn't kill off the cancer altogether, but there is hope a refined process could make that a possibility for the future.
More research is also needed to see if this can work as well in humans as it does in mice, but as these were human cancers xenografted to mice, the work so far is much more promising than a traditional mouse study.
"[T]he genome approach described here should in principle be applicable to most human cancers carrying fusion genes," the researchers conclude.
The study has been published in Nature Biotechnology.
SOURCE
Attachments
- Messages
- 39
- Reaction score
- 0
- Points
- 26
salamuch sa info ts..
Last edited:
- Messages
- 2,145
- Reaction score
- 5
- Points
- 28
- Thread Starter
- #78
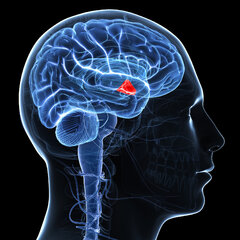
AGING: The fountain of youth in our heads all along?

As far as brain regions go, the hypothalamus is something of a multitasker: it helps control our temperature, hunger, sleep, emotions, and sex drive.
But that's not all. A new study suggests it's also responsible for keeping us young, thanks to a supply of neural stem cells that regulate our ageing.
Sadly, these disappear with time – which could be why we get old – but tests with mice show that implanting new cells to replace them can actually extend lifespan.
"Our research shows that the number of hypothalamic neural stem cells naturally declines over the life of the animal, and this decline accelerates ageing," says molecular pharmacologist Dongsheng Cai from the Albert Einstein College of Medicine in the Bronx.
"But we also found that the effects of this loss are not irreversible. By replenishing these stem cells or the molecules they produce, it's possible to slow and even reverse various aspects of ageing throughout the body."
Cai and his team discovered back in 2013 that the hypothalamus plays a role in ageing, and that by reducing inflammation in the brains of mice, the animals were able to live longer lives.
Now, in a follow-up study, the researchers think they've pinpointed the particular cells in the hypothalamus that matter here: neural stem cells, which serve to generate replacements for dead and damaged cells.
In mice, these cells start to disappear when the animals are about 10 months old (mice middle age), and are largely gone by the time they turn two (elderly).
To figure out if this reduction is what helps cause ageing – as opposed to just a correlation – the researchers disrupted the neural stem cells in a group of mice, using a toxin to destroy around 70 percent of the cells.
Doing so not only caused the mice to live a few months less than naturally ageing control animals, but it also increased the effects of ageing while they still lived.
"There was a decline in learning and memory, coordination, muscle mass, endurance, and skin thickness," Cai explained to Jessica Hamzelou at New Scientist.
To see if an opposite effect was also possible – in other words, whether stocking up on neural stem cells could produce youthful vigour and longevity – the team injected hypothalamic stem cells taken from newborn mice into the brains of two groups of mice.
One of these groups was made up of normal old mice; the other consisted of animals that had had their hypothalamus disrupted by the toxin. The treated animals lived significantly longer than untreated animals, enjoying a lifespan up to 15 percent longer than the controls.
The team thinks that the longevity provided by these neural stem cells comes down to molecular secretions called microRNAs (miRNAs), which help to regulate gene expression.
Scientists uninvolved with the research have described this ageing mechanism as "totally novel and quite unexpected", although there's no guarantee the same physiological function is at work in people.
Finding out whether it is will be on the horizon for the researchers, who now want to launch clinical trials to see if neural stem cells implanted into human volunteers acts like some kind of elixir of youth.
"Of course humans are more complex," Cai explained to Ian Sample at The Guardian.
"However, if the mechanism is fundamental, you might expect to see effects when an intervention is based on it."
The findings are reported in Nature.
Attachments
GENETICS: WE ARE ALL AFRICANSSUMMARY
by George Busby |October 31, 2016
Three new studies reveal the diversity of early human populations and help pin down when we left Africa.
- All humans across the globe right now—no exception—trace their roots to Africa.
[*]Tracing the ancestor of all humans on Earth is a direct consequence of the Human Genome Project (HGP), the international scientific research project with the goal of determining the sequence of nucleotide base pairs which make up human DNA, and of identifying and mapping all of the genes of the human genome from both a physical and a functional standpoint.
[*]Genetic and paleontological evidence show humans only started to leave Africa between 60,000 and 70,000 years ago.
[*]What set this in motion is a major cataclysm, something to do with major climatic shifts that were happening around that time—a sudden cooling in the Earth’s climate driven by the onset of one of the worst parts of the last Ice Age.- The last of our African ancestors who managed to survive and escape Africa were reduced to a population as low as 10,000—humanity was holding on by a thread at this time. It's the ultimate drama of human existence, one to stand for ages to come.
[*]Genetics will revolutionize human societies in many ways not easy to imagine right now.
Humans are a success story like no other. We are now living in the “Anthropocene” age, meaning much of what we see around us has been made or influenced by people. Amazingly, all humans alive today – from the inhabitants of Tierra del Fuego on the southern tip of the Americas to the Sherpa in the Himalayas and the mountain tribes of Papua New Guinea – came from one common ancestor.
We know that our lineage arose in Africa and quickly spread to the four corners of the globe. But the details are murky. Was there just one population of early humans in Africa at the time? When exactly did we first leave the continent and was there just one exodus? Some scientists believe that all non-Africans today can trace their ancestry back to a single migrant population, while others argue that there were several different waves of migration out of Africa.
Now, three new studies mapping the genetic profiles of more than 200 populations across the world, published in Nature, have started to answer some of these questions.
http://www.symbianize.com/attachment.php?attachmentid=1162959&stc=1Aubrey Lynch, elder from the Wongatha Aboriginal language group, participated in one of the studies.
Image via Preben Hjort, Mayday Film.
Out of Africa
Humans initially spread out of Africa through the Middle East, ranging further north into Europe, east across Asia and south to Australasia. Later, they eventually spread north-east over the top of Beringia into the Americas. We are now almost certain that on their way across the globe, our ancestors interbred with at least two archaic human species, the Neanderthals in Eurasia, and the Denisovans in Asia.
Genetics has been invaluable in understanding this past. While hominin fossils hinted that Africa was the birthplace of humanity, it was genetics that proved this to be so. Patterns of genetic variation – how similar or different people’s DNA sequences are – have not only shown that most of the diversity we see in humans today is present within Africa, but also that there are fewer differences within populations the further you get from Africa.
These observations support the “Out of Africa” model; the idea that a small number of Africans moved out of the continent – taking a much reduced gene-pool with them. This genetic bottleneck, and the subsequent growth of non-African populations, meant that there was less genetic diversity to go round, and so there are fewer differences, on average, between the genomes of non-Africans compared to Africans.
When we scan two genomes to identify where these differences, or mutations, lie, we can estimate how long in the past those genomes split from each other. If two genomes share long stretches with no differences, it’s likely that their common ancestor was in the more recent past than the ancestor of two genomes with shorter shared stretches. By interrogating the distribution of mutations between African and non-African genomes, two of the papers just about agree that the genetic bottleneck caused by the migration out of Africa occurred roughly 60,000 years ago. This is also broadly in line with dating from archaeological investigations.
Their research also manages to settle a long-running debate about the structure of African populations at the beginning of the migration. Was the small group of humans who left Africa representative of the whole continent at that time, or had they split off from more southerly populations earlier?
http://www.symbianize.com/attachment.php?attachmentid=1162961&stc=1SGDP model of the relationships among diverse humans (select ancient samples are shown in red) that fits the data.
Image via Swapan Mallick, Mark Lipson and David Reich.
The Simons Genome Diversity Project compared the genomes of 142 worldwide populations, including 20 from across Africa. They conclusively show that modern African hunter-gatherer populations split off from the group that became non-Africans around 130,000 years ago and from West Africans around 90,000 years ago. This indicates that there was substantial substructure of populations in Africa prior to the wave of migration. A second study, led by Danish geneticist Eske Willersev, with far fewer African samples, used similar methods to show that divergence within Africa also started before the migration, around 125,000 years ago.
More migrations?
Following the move out of the continent, the pioneers must then have journeyed incredibly quickly to Australia. The Danish study, the most comprehensive analysis of Aboriginal Australian and Papuan genomes to date, is the first to really examine the position of Australia at the end of the migration.
They found that the ancestors of populations from “Sahul” – Tasmania, Australia and New Guinea – split from the common ancestor of Europeans and Asians 51,000-72,000 years ago. This is prior to their split from each other around 29,000-55,000 years ago, and almost immediately after the move out of Africa. This implies that the group of people who ended up in the Sahul split away from others almost as soon as the initial group left Africa. Substantial mixing with Denisovans is only seen in Sahulians, which is consistent with this early split.
Crucially, because the ancestors of modern-day Europeans and Asians hadn’t split in two at this point, we think that they must have still been somewhere in western Eurasia at this point. This means that there must have been a second migration from west Eurasia into east Asia later on. The Simons Genome Diversity Project study, by contrast, albeit with a far smaller sample of Sahulian genomes, found no evidence for such an early Sahulian split. It instead shows that the ancestors of East Asians and Sahulians split from western Eurasians before they split from each other, and therefore that Denisovan admixture occurred after the former split from each other.
http://www.symbianize.com/attachment.php?attachmentid=1162962&stc=1A graphic representation of the interaction between modern and archaic human lines, showing traces of an early out of Africa (xOoA) expansion within the genome of modern Sahul populations. Image via Dr. Mait Metspalu, Estonian Biocentre.
Meanwhile, a third paper proposes an earlier, “extra” migration out of Africa, some 120,000 years ago. This migration is only visible in the genomes of a separate set of Sahulians sequenced as part of the Estonian Biocentre Human Genome Diversity Panel. Only around 2% per cent of these genomes can be traced to this earlier migration event, which implies that this wave can’t have many ancestors left in the present day. If true (the two other papers find little support for it), this suggests that there must have been a migration across Asia prior to the big one about 60,000 years ago, and that anatomically modern human populations left Africa earlier than many think.
Whatever the reality of the detail of the Out of Africa event, these studies provide some benchmarks for the timings of some of the key events. Importantly, they are also a huge resource of over 600 new and diverse human genomes that provide the genomics community with the opportunity for further understanding of the paths our ancestors took towards the Anthropocene.
George Busby, Research Associate in Statistical Genomics, University of Oxford
This article was originally published on The Conversation. Read the original article.
When humans first ventured out of Africa some 60,000 years ago, they left genetic footprints still visible today. By mapping the appearance and frequency of genetic markers in modern peoples, we create a picture of when and where ancient humans moved around the world. These great migrations eventually led the descendants of a small group of Africans to occupy even the farthest reaches of the Earth.
Our species is an African one: Africa is where we first evolved, and where we have spent the majority of our time on Earth. The earliest fossils of recognizably modern Homo sapiens appear in the fossil record at Omo Kibish in Ethiopia, around 200,000 years ago. Although earlier fossils may be found over the coming years, this is our best understanding of when and approximately where we originated.
According to the genetic and paleontological record, we only started to leave Africa between 60,000 and 70,000 years ago. What set this in motion is a major cataclysm, something to do with major climatic shifts that were happening around that time—a sudden cooling in the Earth’s climate driven by the onset of one of the worst parts of the last Ice Age. This cold snap would have made life difficult for our African ancestors, and the genetic evidence points to a sharp reduction in population size around this time. In fact, the human population likely dropped to fewer than 10,000. We were holding on by a thread.
Once the climate started to improve, after 70,000 years ago, we came back from this near-extinction event. The population expanded, and some intrepid explorers ventured beyond Africa. The earliest people to colonize the Eurasian landmass likely did so across the Bab-al-Mandab Strait separating present-day Yemen from Djibouti. These early beachcombers expanded rapidly along the coast to India, and reached Southeast Asia and Australia by 50,000 years ago. The first great foray of our species beyond Africa had led us all the way across the globe.
Slightly later, a little after 50,000 years ago, a second group appears to have set out on an inland trek, leaving behind the certainties of life in the tropics to head out into the Middle East and southern Central Asia. From these base camps, they were poised to colonize the northern latitudes of Asia, Europe, and beyond.
Around 20,000 years ago a small group of these Asian hunters headed into the face of the storm, entering the East Asian Arctic during the Last Glacial Maximum. At this time the great ice sheets covering the far north had literally sucked up much of the Earth’s moisture in their vast expanses of white wasteland, dropping sea levels by more than 300 feet. This exposed a land bridge that connected the Old World to the New, joining Asia to the Americas. In crossing it, the hunters had made the final great leap of the human journey. By 15,000 years ago they had penetrated the land south of the ice, and within 1,000 years they had made it all the way to the tip of South America. Some may have even made the journey by sea.
The story doesn’t end there, of course. The rise of agriculture around 10,000 years ago—and the population explosion it created—has left a dramatic impact on the human gene pool. The rise of empires, the astounding oceangoing voyages of the Polynesians, even the extraordinary increase in global migration over the past 500 years could all leave traces in our DNA. There are many human journey questions waiting to be asked and answered.
What stories are waiting to be told in your own DNA?
I wondered what happined in Atlantis
- Messages
- 2,145
- Reaction score
- 5
- Points
- 28
- Thread Starter
- #80
I wondered what happined in Atlantis
A recent work about the Mayans of South America and the researches of Richard Cassaro strongly suggest an ancient civilization that predates all the global cultures we know today, using the unified symbols in most of prominent civilizations from all over the word, a common origin some thousands of years back in mankind's history.
Last edited:
Similar threads
- Replies
- 0
- Views
- 34
- Replies
- 0
- Views
- 194